Explore the question: ‘Explain tropospheric pollution’ Dive into the answers and implications. Let’s unravel together.
Explain Tropospheric Pollution:
Tropospheric pollution refers to the presence of undesirable substances in the troposphere, the Earth’s lowest atmospheric layer. Major air pollutants include nitrogen oxides, sulfur oxides, carbon compounds, and hydrocarbons, leading to various environmental and health issues.
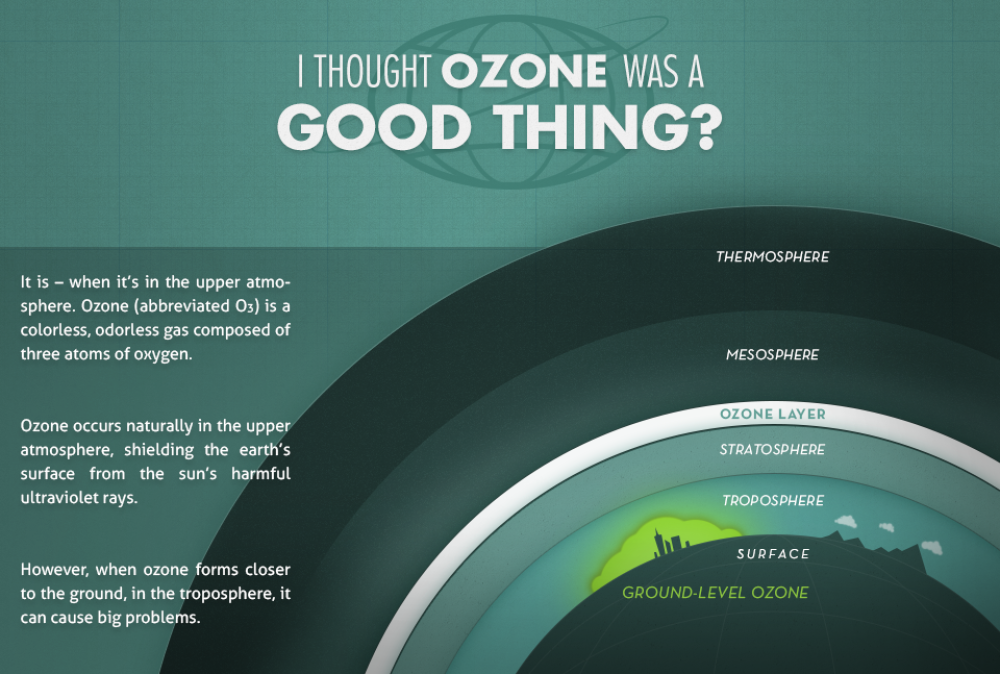
The Earth’s atmosphere is composed of layers based on temperature. These layers, increasing in height, include the troposphere, stratosphere, mesosphere, and thermosphere. Additionally, a further region located approximately 500 kilometers above the Earth’s surface is known as the exosphere.
Sulfur Oxides (SOx):
- When fossil fuels, which contain sulfur compounds, are burned, sulfur dioxide (SO2) is one of the byproducts released into the atmosphere. Sulfur dioxide is a toxic gas and can lead to various respiratory diseases even at low concentrations. It is a major component of air pollution, contributing to the formation of smog and acid rain.
2SO2 (g) + O2 (g) → 2SO3 (g)
- In the presence of oxygen and particulate matter (often acting as catalysts), sulfur dioxide can undergo further oxidation to form sulfur trioxide (SO3). Sulfur trioxide is also a pollutant and contributes to the formation of acid rain when it reacts with atmospheric moisture to produce sulfuric acid (H2SO4).
Formation of Acid Rain:
- Process: Oxides of nitrogen (NO2, NO) and sulfur (SO2, SO3) are released from burning fossil fuels.
- Reaction: They react with water and atmospheric oxygen to form nitric acid (HNO3) and sulfuric acid (H2SO4).
- Result: Acid rain, which harms plants, agriculture, and causes respiratory problems in humans.
| Pollutant | Source | Effect |
|---|---|---|
| NOx | Automobiles, burning fuels | Acid rain, respiratory issues |
| SOx | Burning of coal, fuels | Acid rain, harm to plants and agriculture |
Hydrocarbons: Compounds containing carbon and hydrogen.
- Effect: Combustion of hydrocarbons produces carbon oxides (CO and CO2), contributing to air pollution. Hydrocarbons are carcinogenic in nature and are also regarded as major pollutants.
| Hydrocarbon | Effect |
|---|---|
| Combustion | Oxides of carbon, carcinogenic |
Carbon Monoxide (CO) and Carbon Dioxide (CO2):
- Effect: Reacts with hemoglobin, causing poisoning; contributes to global warming. While carbon dioxide (CO2) is non-toxic, its presence contributes to global warming by trapping additional sunlight. This leads to a warming effect on the Earth, causing an increase in overall temperature.
| Pollutant | Effect |
|---|---|
| CO | Poisonous, contributes to warming |
Particulates: Solid or liquid particles like dust, smoke, fume, and mist.
- Effect: Harmful to health, blocking nasal passages.
| Particulate | Effect |
|---|---|
| Dust, Smoke | Respiratory issues |
Smog:
- Smog is formed through the amalgamation of smoke and fog, leading to reduced visibility for traffic.
- Effect: Photochemical smog, containing ozone, PAN, acrolein, and formaldehyde, causes health issues.
| Smog Type | Components | Effect |
|---|---|---|
| Ordinary (Smoke + Fog) | Reduced visibility | Traffic issues |
| Photochemical | Ozone, PAN, acrolein, formaldehyde | Headaches, eye irritation, chest pain |
Ozone (O3):
- Ozone is beneficial when it is in the upper atmosphere, particularly in the stratosphere. There, ozone molecules form the ozone layer, which plays a crucial role in protecting the Earth’s surface from the harmful effects of ultraviolet (UV) radiation from the sun. UV radiation can cause various health issues such as skin cancer, cataracts, and immune system suppression.
- However, when ozone is present closer to the ground, in the troposphere (the lowest layer of the Earth’s atmosphere), it can contribute to air pollution and pose health risks. In the troposphere, ozone is considered a pollutant and a component of smog.
- It forms through complex chemical reactions involving pollutants emitted from vehicles, industrial processes, and other sources. Ground-level ozone can irritate the respiratory system, exacerbate asthma and other lung conditions, and contribute to cardiovascular problems.
Following table provides a summarized overview of tropospheric pollution, detailing major pollutants, their sources, and associated effects.
| Pollutant Type | Major Sources | Effects |
|---|---|---|
| Nitrogen Oxides (NOx) | Vehicles, burning fuels | Acid rain, respiratory problems, environmental harm |
| Sulfur Oxides (SOx) | Burning of coal, fuels | Acid rain, harm to plants and agriculture |
| Hydrocarbons | Combustion processes | Oxides of carbon, carcinogenic nature |
| Carbon Monoxide (CO) | Vehicle exhaust, burning processes | Poisonous, contributes to global warming |
| Particulates | Industrial processes, combustion | Respiratory issues, health hazards |
| Smog | Combustion processes, industrial activities | Reduced visibility, health issues (photochemical smog) |
Stratospheric Pollution:
The presence of CFCs in the atmosphere leads to the destruction of ozone molecules in the stratosphere, posing significant risks to human health and the environment. This highlights the importance of regulating and phasing out the use of ozone-depleting substances to protect the ozone layer and mitigate the impacts of stratospheric pollution.
- Ozone Formation: In the stratosphere, UV radiation from the sun breaks down dioxygen molecules (O2) into free oxygen atoms (O). These oxygen atoms then combine with dioxygen molecules to form ozone (O3).
O2 + UV → 2O
O + O2 → O3
- Ozone Depletion: Ozone is unstable and can break down back into oxygen molecules. Chlorofluorocarbons (CFCs) are synthetic compounds used in various industrial applications like refrigerants and aerosol propellants. When released into the atmosphere, CFCs rise to the stratosphere, where they are broken down by UV radiation, releasing chlorine atoms.
CFCs + UV → Chlorine Atoms
- Chlorine atoms then react with ozone molecules, leading to the formation of oxygen molecules and chlorine monoxide radicals.
Cl + O3 → ClO + O2
ClO + O → Cl + O2
- Consequences of Ozone Depletion: The depletion of the ozone layer is concerning because ozone acts as a protective shield, absorbing harmful UV radiation from the sun. UV radiation can cause various health problems, including skin cancer and cataracts, and can also harm marine ecosystems and agriculture.


